Tissue Engineering
Silk fibroin bioink for 3D printing in tissue regeneration
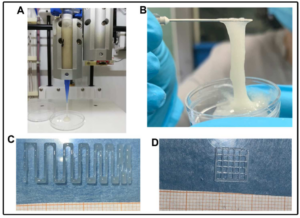 Sodium alginate (SA)-based hydrogels are often employed as bioink for three-dimensional (3D) scaffold bioprinting. They offer a suitable environment for cell proliferation and differentiation during tissue regeneration and also control the release of growth factors and mesenchymal stem cell secretome, which is useful for scaffold biointegration. However, such hydrogels show poor mechanical properties, fast-release kinetics, and low biological performance, hampering their successful clinical application. In this work, silk fibroin (SF), a protein with excellent biomechanical properties frequently used for controlled drug release, was blended with SA to obtain improved bioink and scaffold properties. Firstly, we produced a printable SA solution containing SF capable of the conformational change from Silk I (random coil) to Silk II (β-sheet): this transition is a fundamental condition to improve the scaffold’s mechanical properties. Then, the SA-SF blends’ printability and shape fidelity were demonstrated, and mechanical characterization of the printed hydrogels was performed: SF significantly increased compressive elastic modulus, while no influence on tensile response was detected. Finally, the release profile of Lyosecretome—a freeze-dried formulation of MSC-secretome containing extracellular vesicles (EV)—from scaffolds was determined: SF not only dramatically slowed the EV release rate, but also modified the kinetics and mechanism release with respect to the baseline of SA hydrogel. Overall, these results [1] lay the foundation for the development of SA-SF bioinks with modulable mechanical and EV-release properties, and their application in 3D scaffold printing.
Sodium alginate (SA)-based hydrogels are often employed as bioink for three-dimensional (3D) scaffold bioprinting. They offer a suitable environment for cell proliferation and differentiation during tissue regeneration and also control the release of growth factors and mesenchymal stem cell secretome, which is useful for scaffold biointegration. However, such hydrogels show poor mechanical properties, fast-release kinetics, and low biological performance, hampering their successful clinical application. In this work, silk fibroin (SF), a protein with excellent biomechanical properties frequently used for controlled drug release, was blended with SA to obtain improved bioink and scaffold properties. Firstly, we produced a printable SA solution containing SF capable of the conformational change from Silk I (random coil) to Silk II (β-sheet): this transition is a fundamental condition to improve the scaffold’s mechanical properties. Then, the SA-SF blends’ printability and shape fidelity were demonstrated, and mechanical characterization of the printed hydrogels was performed: SF significantly increased compressive elastic modulus, while no influence on tensile response was detected. Finally, the release profile of Lyosecretome—a freeze-dried formulation of MSC-secretome containing extracellular vesicles (EV)—from scaffolds was determined: SF not only dramatically slowed the EV release rate, but also modified the kinetics and mechanism release with respect to the baseline of SA hydrogel. Overall, these results [1] lay the foundation for the development of SA-SF bioinks with modulable mechanical and EV-release properties, and their application in 3D scaffold printing.
Collaborations
- M. Torre, E. Bari, S. Perteghella – Dept. Drug Science, University of Piemonte Orientale
- S. Perteghella – Dept. Drug Science, University of Pavia
- M. Sorlini – SUPSI—Department of Innovative Technologies, Lugano University Centre
References
[1] E. Bari, G.M. Di Gravina, F. Scocozza, S. Perteghella, B. Frongia, S. Tengattini, L. Segale, M. Sorlini, M.L. Torre, and M. Conti. “Silk Fibroin Bioink for 3D Printing in Tissue Regeneration: Controlled Release of MSC extracellular Vesicles”. Pharmaceutics (2023) – doi: 10.3390/pharmaceutics15020383 – Open Access article under the CC BY license.
3D Bioprinted Scaffolds Containing Lyosecretome
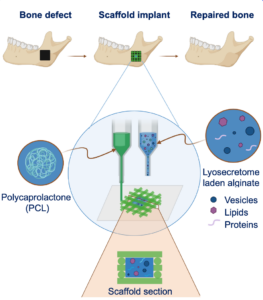 The in vivo grafting of a 3D-printed scaffold does not always end successfully: sometimes, the cells do not colonize the scaffold, causing tissue necrosis. Scaffold enrichment with the Mesenchymal Stem Cells secretome (i.e. substances secreted by MSCs) could promote the in vitro proliferation and differentiation of cells seeded on the scaffold and scaffold colonization when it is implanted in vivo.
The in vivo grafting of a 3D-printed scaffold does not always end successfully: sometimes, the cells do not colonize the scaffold, causing tissue necrosis. Scaffold enrichment with the Mesenchymal Stem Cells secretome (i.e. substances secreted by MSCs) could promote the in vitro proliferation and differentiation of cells seeded on the scaffold and scaffold colonization when it is implanted in vivo.
This work proposes the design and manufacturing of 3D-printed polycaprolactone (PCL) scaffolds, enriched with lyosecretome, a freeze-dried formulation of MSC secretome, containing proteins and extracellular vesicles (EVs), intended for the in vivo controlled release secretome in bone regenerative medicine. Thanks to this strategy it is possible to control the lyosecretome release kinetics by changing the hydrogel concentration, crosslinking process, and the scaffold’s shape [1]. Moreover, the scaffold promotes the formation of a mineralized bone extracellular matrix [2]. Such scaffold prototypes are now available for safety and efficacy tests for bone tissue engineering applications.
The product of this activity was covered by a PCT patent-pending formula.
Collaborations
- M. Torre, E. Bari, S. Perteghella – Dept. Drug Science, University of Pavia
- M. Sorlini – SUPSI—Department of Innovative Technologies, Lugano University Centre
- PharmaExceed S.r.l
- P4P S.r.l.
References
[1] E. Bari, F. Scocozza, S. Perteghella, M. Sorlini, F. Auricchio, M.L. Torre, M. Conti. “3D Bioprinted Scaffolds Containing Mesenchymal Stem/Stromal Lyosecretome: Next Generation Controlled Release Device for Bone Regenerative Medicine”, Pharmaceutics (2021) – doi: 10.3390/pharmaceutics13040515 – Open Access article under the CC BY license.
[2] E. Bari, F. Scocozza, S. Perteghella, L. Segale, M. Sorlini, F. Auricchio, M. Conti, M.L. Torre. “Three-Dimensional Bioprinted Controlled Release Scaffold Containing Mesenchymal Stem/Stromal Lyosecretome for Bone Regeneration: Sterile Manufacturing and In Vitro Biological Efficacy”, Biomedicines (2022) – doi: 10.3390/biomedicines10051063 – Open Access article under the CC BY license.
Myoblast 3D bioprinting to burst in vitro skeletal muscle differentiation
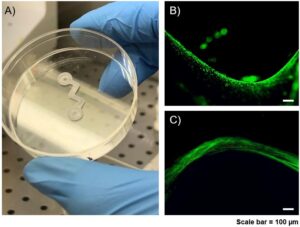 Soft tissue regeneration is a main challenge in tissue engineering applications due to limitations related to vascularization and the restoration of defects with a high volume-to-surface ratio. In particular, skeletal muscle tissues are composed of contractile materials and other important components, like connective tissue, blood vessels, and nerves. When muscle loss becomes irreversible, these damages impair muscle activity.
Soft tissue regeneration is a main challenge in tissue engineering applications due to limitations related to vascularization and the restoration of defects with a high volume-to-surface ratio. In particular, skeletal muscle tissues are composed of contractile materials and other important components, like connective tissue, blood vessels, and nerves. When muscle loss becomes irreversible, these damages impair muscle activity.
The project aims to recapitulate in vitro a fully functional muscle fiber by means of 3D co-printing techniques for manufacturing a 3D construct composed of polycaprolactone (PCL) and a fibrinogen-based hydrogel loaded with murine myoblasts (C2C12) for muscle regeneration. The proposed strategy has produced promising results, however, some limitations, such as long maturation times for the printed fabric and inhomogeneous differentiation, have yet to be overcome. Therefore, the next step will be to apply mechanical stimulation to the co-printed construct by means of a specially designed bioreactor in order to obtain, in the shortest possible time, a fully mature in vitro muscle model.
Collaborations
- G. Cusella, G. Ceccarelli, M. Sampaolesi – Dept. Human Anatomy Unit, Dept. of Public Health, Exp. Medicine and Forensic, University of Pavia
- F. Ronzoni – Humanitas hospital, Milan
- F. Aliberti – IRCCS Policlinico San Matteo, Pavia
3D-printed bioscaffolds for bone transplant
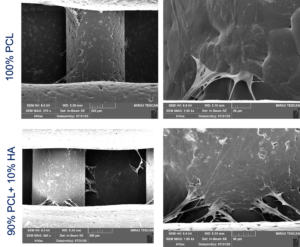 The replacement of bone with artificial materials or with implants of bony tissue taken from other bones of the same patient is not entirely effective due to problems with osteointegration of allograft and the amount of bone available from the body.
The replacement of bone with artificial materials or with implants of bony tissue taken from other bones of the same patient is not entirely effective due to problems with osteointegration of allograft and the amount of bone available from the body.
The project aims at covering such gaps using 3D printing and tissue engineering tools to create patient-specific (under both geometrical and biological viewpoints) biocompatible substitutes for the diseased. To give an effective answer to the preceding clinical problems, we hypothesize a “recipe” with the following main ingredients: i) stem cells of the patient, from which his/her bone cells can be obtained; ii) innovative biomaterials printed in 3D for a custom product dedicated to the patient’s pathology; iii) bioreactors able not only to heal the bone tissue but also to exploit the potential of the patient’s stem cells.
The project foresees also in vivo application testing the bone regeneration ability of the tissue-engineered scaffolds in a sheep model.
Progetto Ricerca Corrente 2018 – IRCCS Policlinico San Matteo
Collaborations
- F. Benazzo, P. Canzi, M. Benazzo, L. Caliogna – IRCCS Policlinico San Matteo, Pavia
- G. Magenes, G. Gastaldi, L. Fassina – CHT – Center of Health Technology, University of Pavia
- V. Bina – Dept. Molecular Biology and Genetics, University of Pavia
